Surprisingly hand-crafted. Outside: a buffed zinc outer unit. Inside: a motor spins a blower wheel at a rate of 20,000 revolutions per minute. See video below.
Rivers and Roads
I’ve set up a Tumblr for our 2012 trip from Nashville to San Francisco. We’re taking the long route. We will be visiting friends and family. I will be swimming in 11 states and posting pictures, video, observations, background research, interviews, and short essays. If you’re interested in keeping up with the trip, please follow! I’ll be cross-posting links to Twitter each week as well.
The Twilight of Moore’s Law?

For decades, the computer industry has been driven by Moore’s law, which started as an observation by Intel’s Gordon Moore that computer chips doubled in capability (or shrink in size by half) every 18 months or so. But at this point, the law has become something of an edict in the industry: improve compute power or bust.
In the shadow of Moore’s law, however, has been another computing trend that’s just now starting to emerge. Based on six decades of data, researchers have found that the energy efficiency of computers also doubles every 18 months.
This power-saving trend, called ‘Koomey’s law’ after Jonathan Koomey of Stanford University who led the study, will have even greater implications than Moore’s law in the coming decades. Consider our love affair with the phone, laptop, and tablet, all battery powered computers. Portability now trumps computing power for many people. In an era where energy efficiency reigns, Moore’s law could become moot.
Here’s my take in Technology Review:
Lasers of Unusual Material
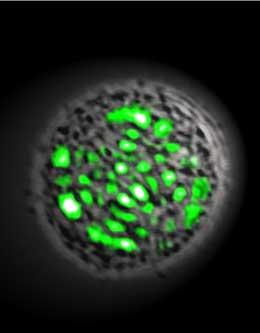
Today’s Nature Photonics story of a laser made of a kidney cell got me thinking of all the weird ways lasers can be made. Such as: they can be made out of a gin and tonic drink, on a stretchy rubber-like material, and they can occur naturally in the atmospheres of Mars and Venus.
What do all of these lasers have in common?
1. A lasing medium. The medium can be a solid, liquid, gas, or plasma in which atoms, molecules, or ions are pumped up to an “excited” energy state. When they relax to a lower energy state, they emit photons. The pumping is usually done by shining light into the medium or using an electric current.
2. A resonant cavity. Bascially, a set of mirrors that let photons bounce back and forth, knocking into excited particles, forcing them down to lower states so they give up photons of a uniform wavelength, moving in a uniform direction.***
In the case of the kidney cell, the lasing medium was a green fluorescent protein (GFP), found in jellyfish to make them bioluminescent. Researchers from Harvard engineered an embryonic kidney cell to produce GFP, placed it between two mirrors 20 microns apart, and pumped it with pulses of blue light. The so-called living laser can’t compete with a laser pointer, but it does produce a green glow that was an order of magnitude brighter than that of a jellyfish. (No cells were damaged in the making of this light emitter.)
But what about an alcoholic drink as a laser? According to Popular Mechanics, researchers at the National Bureau of Standards in Boulder, CO made a number of cocktail lasers in 1975. The Boulder researchers put gin in a copper tube that had two copper mirrors (one with a hole to let light escape) at either end. They then pumped the gin with 20 watts of light from a carbon-dioxide laser. The resulting light was infrared–invisible to the human eye–and quite weak: only 0.00001 watts.
Next up is the rubber laser. A stretchy laser is unusual because most solid lasers are made of inorganic materials, like semiconductor crystals. While organic molecules, which easy bend and stretch are well known light emitters, they’ve only recently been demonstrated as lasers. I recently wrote about a Princeton researcher who showed that a light-emitting coating atop a reflective coating on rubber can produce a red laser that changes its color as the rubber is stretched. It could be useful for sensing structural damage on buildings and bridges.
And finally, the Martian and Venetian lasers in the sky. In 1981, Mumma, et. al. published a paper in Science detailing the analysis of light emitted from Mars. Carbon dioxide in the Martian atmosphere is excited by solar radiation, and emits infrared light with characteristics of a laser, as measured by a spectrometer. Although the paper didn’t say, I’d guess that a resonant cavity could be produced within layers of the atmosphere that have various indices of refraction–the same way light bouncing at particular angles can be trapped between glass and air or water and air. Later, similar emissions also detected on Venus.
——————–
***Also important to making a laser, are a couple of concepts that describe what’s happening inside a lasing medium and between the mirrors:
1. Population inversion. Once a majority of particles are pumped up to the excited state, the medium is said to have reached population inversion, which is special because it’s a requirement for lasers to keep churning out those characteristic laser photons that are all of the same color and moving in the same direction.
2. Stimulated emission. This is the type of emission that happens when photons of a particular wavelength knock those particles sitting in population inversion off their high horse so they emit photons. (The word laser started out as an acronym for Light Amplification by Stimulated Emission of Radiation.)
——————–
Related links:
Nature News article about the living laser: http://www.nature.com/news/2011/110612/full/news.2011.365.html
Popular Mechanics article about gin and tonic lasers: http://www.popularmechanics.com/science/energy/next-generation/how-to-make-a-laser-from-a-gin-and-tonic
Technology Review article on rubber lasers: http://www.technologyreview.com/computing/32336/
Martian atmospheric laser in Science: http://www.sciencemag.org/content/212/4490/45.full.pdf?sid=b5a3470a-9fd5-46fc-9f4a-62ad1b3adecf
An excellent online source that explains how lasers work: http://repairfaq.ece.drexel.edu/sam/lasersam.htm
Making a Little Hydrogen
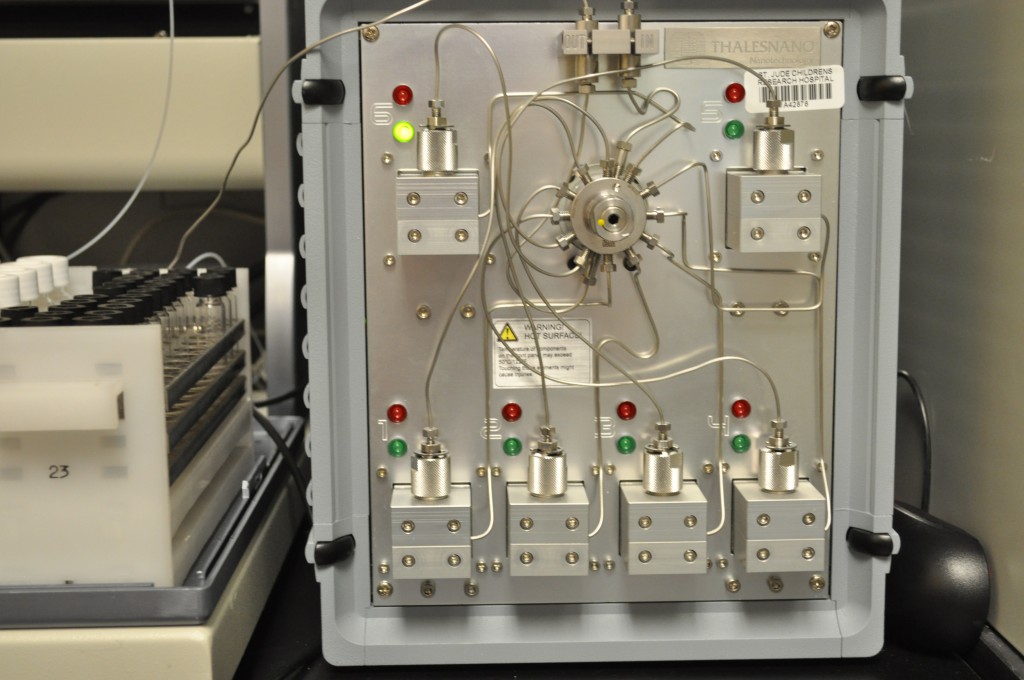
This hydrogen generator is used to make small amounts of hydrogen for chemical reactions in the analytical chemistry lab at St. Jude Children’s Research Hospital, where scientists are searching for compounds that disrupt the growth of childhood cancers.
There was a time when chemistry labs stored large amounts of hydrogen–which is highly flammable when mixed with air–in tanks inside a facility. But small hydrogen generators now let chemists make as little or as much hydrogen as they need for a particular reaction. It’s less wasteful, and a lot safer, cheaper, and cuter.

Not as cute.